Free-Standing PVDF/Reduced Graphene Oxide Film for All-Solid-State Flexible Supercapacitors towards Self-Powered Systems
Abstract
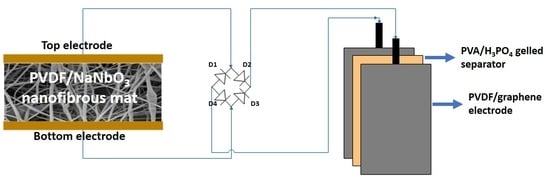
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sodium Niobate Nanocubes
2.3. Electrospinning of Polyvinylidene Fluoride (PVDF)/Sodium Niobate Nanofibers
2.4. Preparation of Graphene Nanosheets
2.5. Preparation and Fabrication of PVDF/Reduced Graphene Oxide Solid-State Supercapacitor
2.6. Instrumentation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.; Wang, Z.; Yuan, R.; Lin, Y.; Yan, J.; Zhang, J.; Lu, Z.; Luo, D.; Pietrasik, J.; Bockstaller, M.R.; et al. ZnO/carbon hybrids derived from polymer nanocomposite precursor materials for pseudocapacitor electrodes with high cycling stability. Polymer 2018, 137, 370–377. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Rajkumar, M.; Rajavel, K.; Zhu, P.; Xuan, W.; Xu, Z.-X.; Wang, F. Porous nickel oxide microsphere and Ti3C2Tx hybrid derived from metal-organic framework for battery-type supercapacitor electrode and non-enzymatic H2O2 sensor. Electrochim. Acta 2019, 322, 134771. [Google Scholar] [CrossRef]
- Veluswamy, P.; Sathiyamoorthy, S.; Khan, F.; Ghosh, A.; Abhijit, M.; Hayakawa, Y.; Ikeda, H. Incorporation of ZnO and their composite nanostructured material into a cotton fabric platform for wearable device applications. Carbohydr. Polym. 2017, 157, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Synthesis of copper benzene-1, 3, 5-tricarboxylate metal organic frameworks with mixed phases as the electrode material for supercapacitor applications. Appl. Surf. Sci. 2018, 460, 33–39. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S.J. Understanding the Thermal Treatment Effect of Two-Dimensional Siloxene Sheets and the Origin of Superior Electrochemical Energy Storage Performances. ACS Appl. Mater. Interfaces 2019, 11, 624–633. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Kim, S.-J. Two-dimensional molybdenum diselenide nanosheets as a novel electrode material for symmetric supercapacitors using organic electrolyte. Electrochim. Acta 2019, 295, 591–598. [Google Scholar] [CrossRef]
- Lisowska-Oleksiak, A.; Wilamowska, M.; Jasulaitiene, V. Organic–inorganic composites consisted of poly(3,4-ethylenedioxythiophene) and Prussian Blue analogues. Electrochim. Acta 2011, 56, 3626–3632. [Google Scholar] [CrossRef]
- Venugopal, G.; George, R.; Raghavan, N.; Srinivas, T.; Dakshinamurthy, A.; Paul, A.J.; Marahatta, A.B. Structural and Mechanical Properties of MgO-Poly(Vinyl Alcohol) Nanocomposite Film. Adv. Sci. Eng. Med. 2015, 7, 457–464. [Google Scholar] [CrossRef]
- Pu, X.; Li, L.; Liu, M.; Jiang, C.; Du, C.; Zhao, Z.; Hu, W.; Wang, Z.L. Wearable Self-Charging Power Textile Based on Flexible Yarn Supercapacitors and Fabric Nanogenerators. Adv. Mater. 2016, 28, 98–105. [Google Scholar] [CrossRef]
- Pandiyarasan, V.; Archana, J.; Pavithra, A.; Ashwin, V.; Navaneethan, M.; Hayakawa, Y.; Ikeda, H. Hydrothermal growth of reduced graphene oxide on cotton fabric for enhanced ultraviolet protection applications. Mater. Lett. 2017, 188, 123–126. [Google Scholar] [CrossRef]
- Hikku, G.; Jeyasubramanian, K.; Venugopal, A.; Ghosh, R. Corrosion resistance behaviour of graphene/polyvinyl alcohol nanocomposite coating for aluminium-2219 alloy. J. Alloys Compd. 2017, 716, 259–269. [Google Scholar] [CrossRef]
- Pandiyarasan, V.; Suhasini, S.; Archana, J.; Navaneethan, M.; Majumdar, A.; Hayakawa, Y.; Ikeda, H.; Abhijit, M. Fabrication of hierarchical ZnO nanostructures on cotton fabric for wearable device applications. Appl. Surf. Sci. 2017, 418, 352–361. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Sahoo, S.; Manoharan, S.; Kim, S.-J. A High Efficacy Self-Charging MoSe2 Solid-State Supercapacitor Using Electrospun Nanofibrous Piezoelectric Separator with Ionogel Electrolyte. Adv. Mater. Interfaces 2018, 5, 1800055. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Ko, T.H.; Seong, J.-G.; Radhakrishnan, S.; Kwak, C.-S.; Khil, M.-S.; Kim, H.-Y.; Kim, B.-S. Dual functional nickel cobalt/MWCNT composite electrode-based electrochemical capacitor and enzymeless glucose biosensor applications: Influence of Ni/Co molar ratio. J. Ind. Eng. Chem. 2019, 73, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of low mass-transfer-resistance fluorinated TiO2 -SiO2/PVDF composite hollow fiber membrane used for biogas upgrading in gas-liquid membrane contactor. J. Membr. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Spreafico, M.A.; Cojocaru, P.; Magagnin, L.; Triulzi, F.; Apostolo, M. PVDF Latex As a Binder for Positive Electrodes in Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2014, 53, 9094–9100. [Google Scholar] [CrossRef]
- Akashi, N.; Kuroda, S.-I. Protein immobilization onto poly (vinylidene fluoride) microporous membranes activated by the atmospheric pressure low temperature plasma. Polymer 2014, 55, 2780–2791. [Google Scholar] [CrossRef] [Green Version]
- Parida, K.; Bhavanasi, V.; Kumar, V.; Wang, J.; Lee, P.S. Fast charging self-powered electric double layer capacitor. J. Power Sources 2017, 342, 70–78. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Mariappan, V.K.; Pazhamalai, P.; Sahoo, S.; Kim, S.-J. Mechanical energy harvesting properties of free-standing carbyne enriched carbon film derived from dehydrohalogenation of polyvinylidene fluoride. Nano Energy 2019, 59, 453–463. [Google Scholar] [CrossRef]
- Xing, L.; Nie, Y.; Xue, X.; Zhang, Y. PVDF mesoporous nanostructures as the piezo-separator for a self-charging power cell. Nano Energy 2014, 10, 44–52. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, M.; Hong, J.-I.; Ding, Y.; Chen, C.-Y.; Chou, L.-J.; Wang, Z.L. Lead-Free NaNbO3 Nanowires for a High Output Piezoelectric Nanogenerator. ACS Nano 2011, 5, 10041–10046. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Zhu, G.; Shang, T.; Xu, Z.; Sun, J.; Zhou, Q. PVP-mediated synthesis of MPO4 (M = Y, Er) hollow mesocrystal cubes via a ripening process. CrystEngComm 2012, 14, 6540. [Google Scholar] [CrossRef]
- Nawaz, M.; Almofty, S.A.; Qureshi, F. Preparation, formation mechanism, photocatalytic, cytotoxicity and antioxidant activity of sodium niobate nanocubes. PLOS ONE 2018, 13, e0204061. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Kim, S.J. Fabrication of high energy Li-ion hybrid capacitor using manganese hexacyanoferrate nanocubes and graphene electrodes. J. Ind. Eng. Chem. 2018, 64, 134–142. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Zang, X.; Cao, Y.; He, Y.; Li, P.; Wang, K.; Wei, J.; Wu, D.; Zhu, H. Effect of different gel electrolytes on graphene-based solid-state supercapacitors. RSC Adv. 2014, 4, 36253–36256. [Google Scholar] [CrossRef]
- Abdelhamid, E.H.; Jayakumar, O.D.; Kotari, V.; Mandal, B.P.; Rao, R.; Naik, V.M.; Naik, R.; Tyagi, A.K. Multiferroic PVDF–Fe3O4 hybrid films with reduced graphene oxide and ZnO nanofillers. RSC Adv. 2016, 6, 20089–20094. [Google Scholar] [CrossRef]
- Shen, Z.X.; Wang, X.B.; Tang, S.H.; Kuok, M.H.; Malekfar, R. High-pressure Raman study and pressure-reduced phase transitions of sodium niobate NaNbO3. J. Raman Spectrosc. 2000, 31, 439–443. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Papila, M. Dielectric behavior characterization of a fibrous-ZnO/PVDF nanocomposite. Polym. Compos. 2009, 31, 1003–1010. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium–Sodium Niobate Lead-Free Piezoelectric Materials: Past, Present, and Future of Phase Boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef]
- He, H.; Fu, Y.; Zang, W.; Wang, Q.; Xing, L.; Zhang, Y.; Xue, X. A flexible self-powered T-ZnO/PVDF/fabric electronic-skin with multi-functions of tactile-perception, atmosphere-detection and self-clean. Nano Energy 2017, 31, 37–48. [Google Scholar] [CrossRef]
- Choi, M.; Murillo, G.; Hwang, S.; Kim, J.W.; Jung, J.H.; Chen, C.-Y.; Lee, M. Mechanical and electrical characterization of PVDF-ZnO hybrid structure for application to nanogenerator. Nano Energy 2017, 33, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lee, K.Y.; Hwang, S.K.; Park, C.; Kim, S.-W.; Cho, J. Layer-by-Layer Controlled Perovskite Nanocomposite Thin Films for Piezoelectric Nanogenerators. Adv. Funct. Mater. 2014, 24, 6262–6269. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kim, D.-I.; Duy, L.T.; Nguyen, M.T.; Muhammad, S.; Yoon, W.-S.; Lee, N.-E. High-performance flexible lead-free nanocomposite piezoelectric nanogenerator for biomechanical energy harvesting and storage. Nano Energy 2015, 15, 177–185. [Google Scholar] [CrossRef]
- Kang, H.B.; Han, C.S.; Pyun, J.C.; Ryu, W.H.; Kang, C.-Y.; Cho, Y.S. (Na,K)NbO3 nanoparticle-embedded piezoelectric nanofiber composites for flexible nanogenerators. Compos. Sci. Technol. 2015, 111, 1–8. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Naebe, M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos. Sci. Technol. 2017, 138, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-I.; Lee, M.; Liu, Y.; Moon, S.; Hwang, G.-T.; Zhu, G.; Kim, J.E.; Kim, S.O.; Kim, D.K.; Wang, Z.L.; et al. Flexible Nanocomposite Generator Made of BaTiO3 Nanoparticles and Graphitic Carbons. Adv. Mater. 2012, 24, 2999–3004. [Google Scholar] [CrossRef]
- Ren, X.; Fan, H.; Zhao, Y.; Liu, Z. Flexible Lead-Free BiFeO3/PDMS-Based Nanogenerator as Piezoelectric Energy Harvester. ACS Appl. Mater. Interfaces 2016, 8, 26190–26197. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Cao, H.; Chang, Y.; Tang, K.; Cao, Z.; Chang, J.; Cao, Y.; Wang, W.; Gao, M.; et al. Carbon materials derived from chitosan/cellulose cryogel-supported zeolite imidazole frameworks for potential supercapacitor application. Carbohydr. Polym. 2017, 175, 223–230. [Google Scholar] [CrossRef]
- Thangavel, S.; Thangavel, S.; Raghavan, N.; Alagu, R.; Venugopal, G.; Raja, A. Efficient visible-light photocatalytic and enhanced photocorrosion inhibition of Ag2WO4 decorated MoS2 nanosheets. J. Phys. Chem. Solids 2017, 110, 266–273. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. Int. J. Hydrogen Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- Elashmawi, I.; Gaabour, L.H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.-Y.; Zhou, W.-H.; He, L.-Y.; Tang, H.-Y.; Xu, X.-H.; Ouyang, Q.-S.; Shao, J.-J. Micro-corrugated graphene sheet enabled high-performance all-solid-state film supercapacitor. Carbon 2020, 160, 156–163. [Google Scholar] [CrossRef]
- Lai, Y.; Wan, L.; Wang, B. PVDF/Graphene Composite Nanoporous Membranes for Vanadium Flow Batteries. Membranes 2019, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Xu, Q.; Chen, Z.; Rao, W.; Fan, L.; Yuan, Y.; Bai, Z.; Xu, J. An all-solid-state yarn supercapacitor using cotton yarn electrodes coated with polypyrrole nanotubes. Carbohydr. Polym. 2017, 169, 50–57. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Raja, T.S.G.; Purushothaman, S.; Kumar, M.V.; Sushmitha, I. Supercapacitive performances of MnO2 nanostructures grown on hierarchical Cu nano leaves via electrodeposition. Electrochim. Acta 2017, 227, 401–409. [Google Scholar] [CrossRef]
- Huang, P.; Pech, D.; Lin, R.; McDonough, J.K.; Brunet, M.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. On-chip micro-supercapacitors for operation in a wide temperature range. Electrochem. Commun. 2013, 36, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Nallamuthu, G.; Thangavel, S.; Kirubakaran, K.; Vasudevan, V.; Sivalingam, Y.; Venugopal, G. Study of structural and electrochemical properties of La2SrV2O9 perovskites prepared using ball-milling. Appl. Surf. Sci. 2018, 449, 468–473. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R. Electrochemical behaviour of manganese & ruthenium mixed oxide@ reduced graphene oxide nanoribbon composite in symmetric and asymmetric supercapacitor. Appl. Surf. Sci. 2018, 427, 102–111. [Google Scholar]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, N.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sources 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S.J. Mechanically delaminated few layered MoS2 nanosheets based high performance wire type solid-state symmetric supercapacitors. J. Power Sources 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Kang, T. Electrochemical studies of spherically clustered MoS2 nanostructures for electrode applications. J. Alloys Compd. 2015, 634, 104–108. [Google Scholar] [CrossRef]
- Pu, X.; Li, L.; Song, H.; Du, C.; Zhao, Z.; Jiang, C.; Cao, G.; Hu, W.; Wang, Z.L. A Self-Charging Power Unit by Integration of a Textile Triboelectric Nanogenerator and a Flexible Lithium-Ion Battery for Wearable Electronics. Adv. Mater. 2015, 27, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, X.; Chen, H.; Huang, J.; Chen, X.; Han, M.; Su, Z.; Meng, B.; Song, Z.; Zhang, H. Integrated self-charging power unit with flexible supercapacitor and triboelectric nanogenerator. J. Mater. Chem. A 2016, 4, 14298–14306. [Google Scholar] [CrossRef]
- Yi, F.; Wang, J.; Wang, X.; Niu, S.; Li, S.; Liao, Q.; Xu, Y.; You, Z.; Zhang, Y.; Wang, Z.L. Stretchable and Waterproof Self-Charging Power System for Harvesting Energy from Diverse Deformation and Powering Wearable Electronics. ACS Nano 2016, 10, 6519–6525. [Google Scholar] [CrossRef]
- Guo, H.; Yeh, M.-H.; Lai, Y.-C.; Zi, Y.; Wu, C.; Wen, Z.; Hu, C.; Wang, Z.L. All-in-One Shape-Adaptive Self-Charging Power Package for Wearable Electronics. ACS Nano 2016, 10, 10580–10588. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, C.; Wang, Z.; Wang, A.C.; He, J.-H.; Wang, Z.L.; Alshareef, H.N. MXene electrochemical microsupercapacitor integrated with triboelectric nanogenerator as a wearable self-charging power unit. Nano Energy 2018, 45, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-Based Supercapacitors for Self-Powered Nanosystems. Angew. Chem. Int. Ed. 2012, 51, 4934–4938. [Google Scholar] [CrossRef]




| Series No. | Piezoelectric Material | Output Voltage | Reference |
|---|---|---|---|
| 1 | (PAA/OA-BTO NP)n thin films | 2.5 V | [33] |
| 2 | PVDF-TrFE/BiTO | 5.0 V | [34] |
| 3 | PVDF-TrFE/(Na, K)NbO3 | 2.0 V | [35] |
| 4 | PVDF/graphene | 12 V | [36] |
| 5 | BaTiO3+CNT+PDMS | 3.0 V | [37] |
| 6 | PVDF/Sodium niobate | 40 V | This work |
| Series No. | Energy Harvester | Energy Storage | Charing Voltage (mV) | Time | Reference |
|---|---|---|---|---|---|
| 1 | Planar type TENG | Planar supercapacitor | 800 | 3 h | [54] |
| 2 | Planar type TENG | Planar supercapacitor | 250 | 100 s | [55] |
| 3 | Planar type TENG | Kirigami supercapacitor | 50 | 20 s | [56] |
| 4 | Planar type TENG | Micro-supercapacitor | 400 | 20 min | [57] |
| 5 | Planar PENG | Supercapacitor | 2500 | 12 h | [58] |
| 6 | Electrospun PVDF/Sodium niobate PENG | Binder free supercapacitor | 800 | 190 s | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazhamalai, P.; Mariappan, V.K.; Sahoo, S.; Kim, W.Y.; Mok, Y.S.; Kim, S.-J. Free-Standing PVDF/Reduced Graphene Oxide Film for All-Solid-State Flexible Supercapacitors towards Self-Powered Systems. Micromachines 2020, 11, 198. https://doi.org/10.3390/mi11020198
Pazhamalai P, Mariappan VK, Sahoo S, Kim WY, Mok YS, Kim S-J. Free-Standing PVDF/Reduced Graphene Oxide Film for All-Solid-State Flexible Supercapacitors towards Self-Powered Systems. Micromachines. 2020; 11(2):198. https://doi.org/10.3390/mi11020198
Chicago/Turabian StylePazhamalai, Parthiban, Vimal Kumar Mariappan, Surjit Sahoo, Woo Young Kim, Young Sun Mok, and Sang-Jae Kim. 2020. "Free-Standing PVDF/Reduced Graphene Oxide Film for All-Solid-State Flexible Supercapacitors towards Self-Powered Systems" Micromachines 11, no. 2: 198. https://doi.org/10.3390/mi11020198